ABOUT OUR WORK
Our research transcends many military public health and leadership disciplines including preventive medicine, community health, and health promotion in the uniformed services. The featured research below is a snapshot of some of our research projects that align with the Department of Defense's Quadruple Aim: achieve medical readiness, improve the health of our people, enhance the experience of care, and lower our healthcare costs.
DISCOVERY OF HIV-1 ENVELOPE GLYCOPROTEINS ASSOCIATED WITH A BROADLY REACTIVE NEUTRALIZING ANTIBODY RESPONSES
The development of a successful vaccine against HIV-1 infection or a therapeutic vaccine agent capable of preventing HIV-1 disease progression has been a public health goal for over 30 years. Most believe that one of the immune responses that may be required to elicit a protective immune response against HIV-1 infection is the generation of antibodies that are virus neutralizing. The major target of virus-neutralizing antibodies are those reactive to the HIV-1 envelope glycoprotein which has been the cornerstone of vaccine development. But discovering the best version of this highly diverse and variable component of the virus has been an elusive task.
Virus neutralizing antibodies are the principal mechanism for effectiveness of all proven viral vaccines, and the target of HIV-1 neutralizing antibodies is the envelope glycoprotein (Env). HIV-1 Env is complex, multimeric oligomeric structure often referred to as the trimeric Env spike glycoprotein. Each spike glycoprotein unit that makes up the trimer, is a dimer composed of the 120 kd surface protein (gp120) and the 41 kd transmembrane protein (gp41). There are a number of virus-neutralization domains on the HIV-1 Env, but the amino acid compositions of the Env glycoproteins vary substantially from strain to strain, with some of the neutralization domains in regions which tend to vary greatly, while others are in regions which tend to be highly conserved. It is the amino acid sequence variation that is undoubtedly a substantial factor for the variation that is seen in specificity of neutralization sensitivity among HIV-1 virus strains. An extraordinary variety of approaches have been carried out over the past several decades to evaluate HIV-1 Env-based vaccines aimed at inducing broadly cross-reactive neutralizing antibodies, all with limited success. Approaches used have included the administration of Env prepared using various recombinant DNA techniques, synthetic peptides representative of particular structures within Env, live viral vectors that express Env in vivo, covalently linked complexes of Env and virus receptor (CD4), among others.
Gerald V. Quinnan, Jr., MD, RADM (Ret), USPHS, and Professor at USU, initiated studies in the late 1990’s aimed at characterizing the neutralizing antibody responses associated with a unique HIV-1 Env glycoprotein known as R2. The gene encoding this envelope protein was recovered from cells from an HIV-1-infected donor, who had antibodies that neutralized many different primary isolates of HIV-1. Primary isolates are notoriously difficult to neutralize, and sera from infected humans generally neutralize few, or a limited subset of strains of HIV-1. The Env gene from the donor was cloned and the R2 Env was characterized extensively and data formed the foundation of two United States Patents. Research on R2 Env as a vaccine included its testing in mice and monkeys, in collaboration with Dr. Christopher C. Broder at USU, involved use of a viral vector for in vivo R2 Env expression and the administration a form of the R2 Env that had been engineered to be a soluble native-like trimer spike referred to as gp140, similar to the intact protein spike, but is produced by cells engineered to express the protein in a form that retains its antigenic characteristics. Immunization with R2 Env induced neutralizing antibodies with cross-reactivity patterns similar to each other and to the cross-reactivity of the serum from the donor of the R2 Env gene. The sera from the immunized mice and monkeys neutralized HIV-1 strains of the A, B, C, and F subtypes. Additional studies on R2 oligomeric Envs as immunogens included the demonstration of receptor-induced conformation change in purified R2 gp140, and together Dr. Quinnan’s studies formed the basis of USU’s first NIH-sponsored Program Project award from the National Institutes of Allergy and Infectious Diseases.
INDOOR RESIDUAL SPRAYING AGAINST MALARIA
The environmental awakening of the 1960-70s was an important societal milestone for our relationship with nature. Like any struggle, it had casualties. A focus at the start of that awakening was the impact on animals by widespread use of the pesticide DDT. Use plummeted, and global malaria control suffered. USU’s Professor Donald R. Roberts, an accomplished entomologist, methodically built the case that while indiscriminate use of DDT was a problem, carefully applied strategies to spray the inside of dwellings could fight the mosquitoes with little other harm, and drop the burden of malaria. His work changed global policy and saved lives.
Targeted use of DDT and other pesticides in indoor residual spraying strategies to reduce malaria burden while avoiding the community consequences of use. Professor Donald R. Roberts was on the leading edge of this movement. Importantly, he not only conducted field research developing indoor residual spraying techniques and demonstrating their efficacy, he understood the importance of communicating his findings in context towards improved public policy. At the time of this work, Professor Roberts was a faculty member of PMB. He created a legacy of field work in indoor residual spraying and community effects in the Entomology section of the Division of Tropical Public Health that continued for several years by subsequent faculty.
Professor Roberts successfully impacted World Health Organization and other policies on DDT and related pesticides, allowing indoor residual spraying to be fully incorporated in anti-malaria programming. This is believed to have substantially reduced malaria-associated morbidity and mortality and also has positively impacted dengue and other arboviral threat risk management. Additionally, the wider acceptance of indoor residual spraying created market space that facilitated the development of lower toxicity pesticides, relevant for both environmental protection and the challenge of resistance to pesticides.
TRIAL EVALUATING AMBULATORY TREATMENT OF TRAVELERS’ DIARRHEA AND THE DEVELOPMENT OF THE FIRST DEPLOYMENT HEALTH GUIDELINES
Under funding ($2.1M) from the Navy Bureau of Medicine and Surgery Wounded Ill and Injured Program, CAPT Mark S. Riddle and Dr. David Tribble developed the first evidenced-based Deployment Health Guideline on Management of Deployment Diarrhea. This effort, started in 2012, saw the completion of the largest DoD randomized controlled clinical trial completed and culminated in a March 2016 two-day Summit where Tri-Service, British, and external-DoD subject matter experts convened to develop these guidelines. Once integrated into education and training doctrine, a substantial improvement in recovered lost-duty days to this most frequent deployment health infection will be realized.
A leading infectious cause of lost duty days during deployment over the last 60 years has been due to diarrhea. While not life-threatening, the impacts can be incapacitating, and the sheer force of infection, often associated with outbreaks at the very onset of battle, can detrimentally impact mission execution. Despite years of research and discovery of bacterial etiologies accounting for the vast majority of these infections, and effective antibiotic therapy reduces an illness that last 3-5 days down 12 hours or less, multiple provider and Service member surveys indicate that access to such effective treatment and utilization of best evidenced therapy are poor. This recognized gap in the management of this frequent (29% monthly attack rate) warfighter infection was addressed through a 2011 proposal funded by the US Navy Bureau of Medicine and Surgery under the Wounded Ill and Injured program. The funded project included two main aims: (1) execution of a randomized controlled trial of three single-dose loperamide adjuncted regimens for treatment of watery diarrhea with a focus on Africa and Asia where recent data were lacking, and (2) development of a Deployment Health Guideline which would amalgamate the results from this clinical trial with epidemiological and previous clinical trial data to develop an evidenced based guideline to be used in the US Navy and Marine Corps.
Figure 2. The Millennium Cohort Study: contributions to a healthy and fit Force
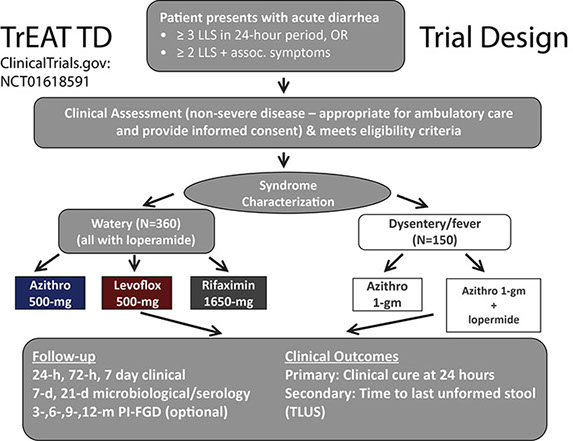
A randomized double-blind clinical trial in collaboration with multiple partners including field sites in Afghanistan and Kenya directed by our United Kingdom Military counterparts, and Army and Navy OCONUS labs in Kenya, Cairo, and Peru who directed field sites in Djibouti and Honduras completed enrollment in July 2015. In this study, entitled the “Trial Evaluating Antibiotic Treatment in Travelers’ Diarrhea (TrEAT TD), 339 US and UK deployed military Service members with acute non-inflammatory diarrhea acquired during combat operational deployment or exercise were randomized to received single dose azithromycin (500 mg), levofloxacin (500 mg), and rifaximin (1650 mg) plus loperamide (labelled dosing). Among these 324 (90.3%) were able to be followed with complete endpoints available. Remarkably, all three arms performed well with clinical cure rates of 75~80% at 24 hours, and an average time to last diarrheal stool of around 15 hours. Of note, this was the first time rifaximin, a safer non-absorbable limited spectrum antibiotic, was used as a single dose providing first evidence of an alternative field expedient antibiotic regimen which may be considered. Such an effectiveness witnessed is remarkable when compared to placebo controlled trials of travelers’ diarrhea (which are no longer performed) where illness on average lasts 60 hours or more (a 75% reduction in illness duration and impacted duty time). Importantly, this trial filled much needed gaps of information on the effectiveness of these regimens in recent times (last DoD trial 1997) and in Africa and Southwest Asia, where there is was a paucity of information. This trial, centrally coordinated by the Infectious Diseases Clinical Research Program at Uniformed Services University was also remarkable in that it provided a real-world operational research opportunity to 69 students, residents, fellows and staff clinicians and allied health providers across the three Services of the US and UK military, and one Canadian. The results were published in Clinical Infectious Diseases Journal in 2017 and can be found here: https://academic.oup.com/cid/article/65/12/2008/4210676
While the TrEAT TD study was the first of its kind in many respects, the sole purpose of this study was to provide information needed to develop globally applicable Deployment Health Guidelines on the Management of Acute Diarrhea. Key clinical questions still needed answered: (1) How should military personnel on deployment with travelers’ diarrhea during deployment be directed with respect to self-care or seeking care?, (2) Which Service members should be prescribed antibiotics to self-treat travelers’ diarrhea?, (3) What antibiotics/regimens should be considered for treatment of acute watery diarrhea?, (4) What antibiotics/regimens should be considered for treatment of febrile diarrhea/dysentery?, (5) When and what laboratory diagnostics should be used to support management of deployment TD? To this aim, a two-day summit was held at the Naval Medical Research Center in Silver Spring, MD in which speakers gave focused presentations on the aforementioned key clinical questions with open dialogue and comment. In attendance were 15 appointed subject matter experts (SMEs) in the fields of infectious diseases, preventive medicine, and gastroenterology; experienced forward deployed physician assistants, Independent Duty Corpsmen, and Medical Technician providers of all three Services; and three external Experts in the field.
The Deployment Health Guideline Summit utilized current best practices as outlined by the Institute of Medicine including the identification and review of the evidence, drafting of the recommendations, evidence grading process, and external review. To avoid groupthink and key opinion leader dominance, an anonymous Delphi process was used to develop and grade all recommendations. This panel developed 13 graded, and 5 ungraded recommendations in the areas of general recommendations on force health protection strategies (2 graded, 3 ungraded), non-antibiotic management of acute diarrheal illness during deployment (2 graded), antibiotic therapy of acute watery diarrheal illness during deployment (5 graded), antibiotic therapy of dysentery and febrile diarrhea (4 graded), and follow-up and diagnostic testing (2 ungraded). A number of critical research gaps were also identified. These recommendations were included as part of a special supplement published in the Military Medicine Journal in the Fall of 2017 can be found here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5657341/
In summary, management of acute diarrheal Illness during deployment requires action at the provider, population and commander levels to be most effective. The execution of a well-designed clinical trial which provided much needed evidence to consider in a comprehensive Deployment Health Guideline development process is the beginning. Effective promulgation and adoption of these guidelines utilizing top-down and bottom up approaches are underway and will require additional commitment on the part of many. However, the effort to date and moving forward are no doubt worth it, as the impact of this common infection continues to degrade our capability, and effective management is a unquestionable necessity until more effective preventive interventions such as vaccines or microbiome modification can be realized.