THE PROBLEM
Our genomes must be maintained accurately from one generation to the next, and one cell division at a time. Otherwise, genome instability can result in aneuploidy, infertility, developmental defects, degenerative diseases, and cancer predisposition. Our research focuses on understanding the molecular mechanisms that monitor DNA replication accuracy and DNA damage repair proficiency, as well as processes that ensure accurate chromosome segregation and cell cycle progression.
OUR APPROACH
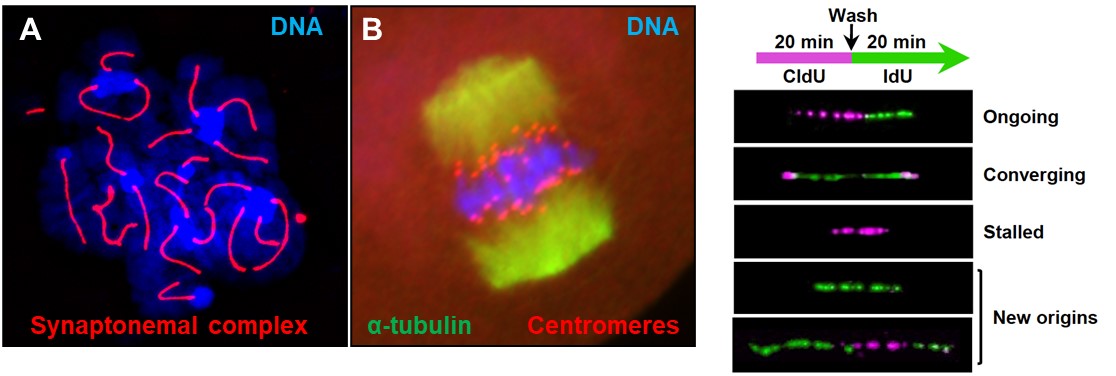
We use mouse and human pluripotent stem cells and mutant mouse models to elucidate key mechanisms required for detecting and combating challenges to genome integrity during gametogenesis (Fig. 1) and embryonic development (Fig. 2 and 3).
|
|
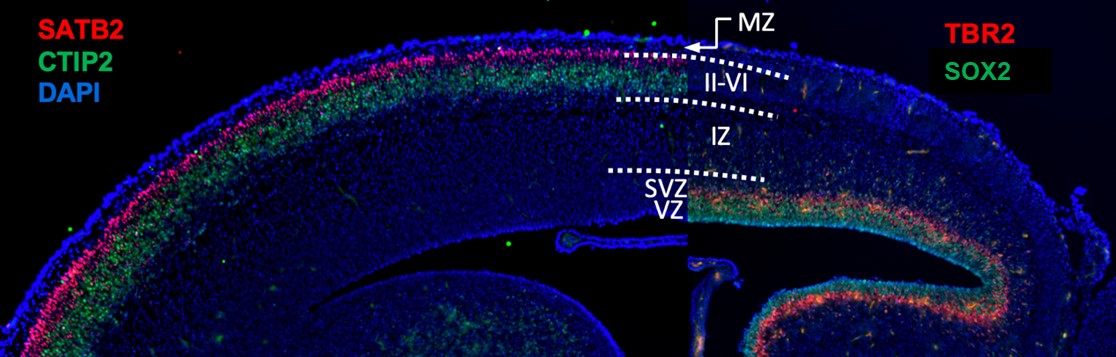
Fig. 3 - Sagittal cross-section of an embryonic mouse brain stained with different classes of neural progenitors and cortical neurons.
TRANSGENIC TECHNOLOGIES
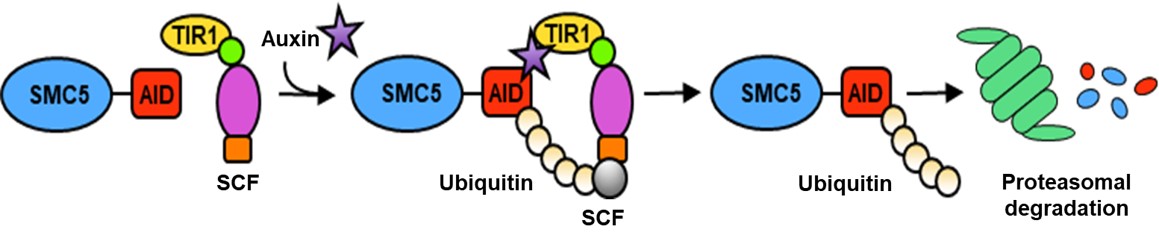
We create new transgenic pluripotent stem cells and animal models for research. For example, we introduced the auxin-inducible degron (AID) system in human and mouse embryonic stem cells and mice. The AID system allows for rapid, titratable, and reversible degradation of a target protein (Fig. 4). This approach is instrumental for mechanistic studies as rapid protein depletion can be stimulated at specific times or stages of development.
Fig. 4 – Targeting the SMC5-AID tagged protein for polyubiquitination and rapid proteasomal degradation.
example research initiatives
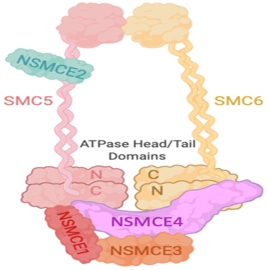
Structural maintenance of chromosomesPluripotent and progenitor stem cells undergo rapid proliferation and are prone to genome instability due to the speed of DNA replication and chromosome segregation. Errors during these processes can result in developmental defects and is also a hallmark of cancer. Our cells safeguard themselves from genomic instability by expressing three classes of structural maintenance of chromosomes (SMC) complexes. We have found that one class, SMC5/6, is critical to ensure DNA replication is completed prior to chromosome segregation. Furthermore, mutations in SMC5/6 components lead to neurodevelopmental defects and aberrant functional connectivity in the brain. We are now using the AID system to analyze the molecular functions of SMC5/6 in embryonic and neural progenitor cells and in post-mitotic neurons. |
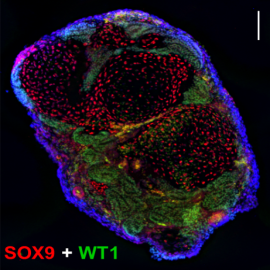
Modeling Human Gonad DevelopmentDuring early stages of development, embryos form a bipotential gonad that, depending on the sex chromosomes, are directed to form the testes or ovaries. Errors in this process can lead to differences in sex development (DSDs). DSD occurs in 1:4500 to 1:5000 live births. Despite this prevalence there is very little known about human gonad development. Therefore, our lab is leading efforts to create human gonad organoids. Modeling Gonad functionFormation of gametes is a sexually dimorphic process. For example, a female is born with a finite number of oocytes, and these are more prone to chromosome missegregation as women age. A male has a non-homologous chromosome pair (X-Y), which are more prone to aneuploidy. Our lab specializes in understanding the mechanisms behind gametogenesis and defects that can arise in humans. |