The Problem
Non-Hodgkin lymphoma and Hodgkin's disease are among the top 10 most frequent cancers in the U.S. general population and in U. S. active duty military personnel. Importantly, some of these lymphomas are caused by infectious agents including Epstein-Barr Virus (EBV), Kaposi’s Sarcoma Herpesvirus (KSHV), and human T-cell leukemia virus type 1 (HTLV-1) that are endemic in Japan, sub-Saharan Africa, Middle East, Mediterranean, and Australo-Melanesia. U.S. military personnel deployed to these regions are at risk for exposure. Indeed, HTLV-1 was first discovered in a cell line, HUT102, derived from the lymphocytes of a patient treated at the VA hospital in Washington, DC. ATL shares many biological characteristics with other NF-kB-addicted lymphoid malignancies including diffuse large B-cell lymphoma, multiple myeloma, chronic lymphocytic leukemia, and EBV- and KSHV-associated lymphoproliferative disorders. Elucidating the mechanisms that underlie ATL development will help illuminate on how other T or B cell malignancies develop and uncover novel biomarkers, treatment targets, and therapeutic strategies. Findings from our work will help guide precision therapeutic approaches to address the cancer health needs of deployed and non-deployed personnel, their dependents, and veterans.
Inside the Lab
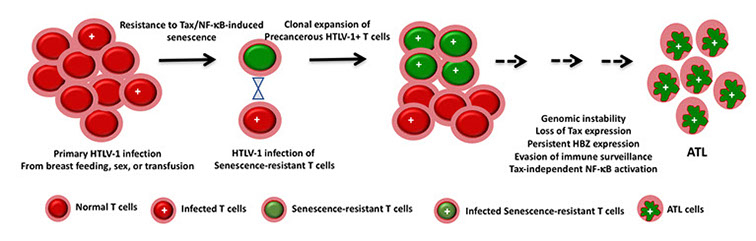
Human T cell leukemia virus type 1 (HTLV-1) is a complex human retrovirus that causes a malignancy of CD4+ T cells known as adult T cell leukemia /lymphoma (ATL). The HTLV-1 regulatory protein, Tax, potently activates viral transcription and the NF-kB/Rel family of transcription factors (referred to as NF-kB) whose activities drive T and B cell proliferation, immune activation, and inflammation. In normal cells, NF-kB activity is stringently regulated and quickly turned off after stimulation. It is however constitutively active in many B- and T-cell malignancies and confers survival and proliferation advantages to cancer cells.
Senescence, a cellular mechanism responsible for tumor suppression, occurs as a result of DNA damage and engagement of the DNA damage response (DDR) pathway and tumor suppressors. We have found that potent and persistent activation of NF-kB by HTLV-1 Tax induces a rapid senescence response (Tax-IRS) mediated by cyclin-dependent kinase inhibitors, p21CIP1/WAF1 (p21) and p27KIP1 (p27). Most HTLV-1-naïve CD4+ T cells or non-lymphoid cells newly infected by HTLV-1 also arrest in senescence. On the contrary, HTLV-1- transformed and patient-derived ATL T cells are invariably resistant to Tax-IRS.. How the transcriptional activity of NF-κB induces cellular senescence and how ATL cells and NF-kB-addicted cancer cells maintain constitutively activated NF-kB without triggering senescence are two of the major problems we are addressing. The figure below depicts our current working hypothesis.
“Presently, slightly >20% of the global cancer incidence has been linked to viral, bacterial, and parasitic infections. Some of these agents act as direct carcinogens, where persistence and expression of viral oncogenes are required to maintain the malignant phenotype of the cancer cells; others act indirectly, e.g., by inducing immunosuppression or chronic inflammation or activating oxygen- or nitroso-radicals.”
Our Approach
We employ a variety of cellular, virological, molecular, and genetic/genomic approaches to address the questions outlined above. In a recent study, we have used cell extracts, purified proteins, and in vitro reconstitution to elucidate the mechanism by which HTLV-1 Tax activates the classical NF-kB pathway. Using lentivirus vectors, we have also derived reporter cell lines to track the outcomes of HTLV-1 infection and senescence induction by the HTLV-1 Tax gene.
Results
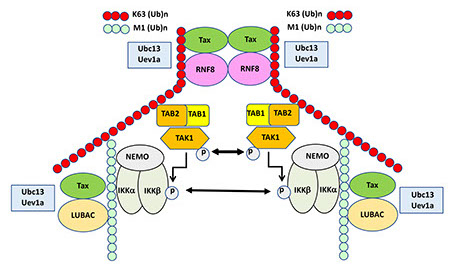
Using in vitro reconstitution in conjunction with shRNA-mediated gene silencing and CRISPR-Cas9-mediated gene ablation , we have discovered that Tax hijacks and aberrantly activates an E3 ubiquitin ligase, RING-finger protein 8 (RNF8), and its associated E2 conjugating enzymes, Ubc13:Uev1a/Uev2, to assemble lysine 63-linked polyubiquitin (K63-pUb) chains, which together with linear polyubiquitin chains, serve as signaling scaffolds to trigger the activation of a kinase cascade involving transforming growth factor beta-activated kinase 1 (TAK1), IkB kinase (IKK), c-Jun N-terminal kinase (JNK), and other kinases.
ur Hendra G glycoprotein subunit vaccine (HeV-sG) is marketed in Australia, manufactured by Zoetis, Inc., (Equivac® HeV) to prevent Hendra virus infection of horses and break the transmission of Hendra infection to people and has been a successful One-Health strategy to counter the transboundary threat posed by Hendra virus infection to both animal and human health.